About
Clinical Trials
What sets up apart?
The BRAS Drug Development Program is Canada’s largest early phase drug academic development program. Our Clinical Trials program has a global impact on cancer research by providing personalized cancer care, giving the right treatment to the right patient at the right time. Our program targets the toughest of cancers.
Clinical trials are important in science and research because they are the most rigorous way to answer two important questions. Is a new drug or a new treatment safe and is a new drug or a new treatment better than the existing standard of care?
Clinical trials help in making critical advances in cancer research. In the last decade we had 5,800 patients enrolled onto 300 clinical trials. On average, 150-160 active trials across multiple disease sites are open at any one time. We have robust infrastructure and specialized expertise in multi-centre clinical trials.
The program has their eye on the horizon with the innate ability to anticipate and adapt to rapidly changing therapeutic development markets. We place a strong focus on mentorship and training of the next generation. We are a strategic outreach to key cancer centres, locally, nationally, and internationally. We are a balanced portfolio of activities and disciplines.
Our focus remains on high quality and timely trials with potential for significant impact. This allows tempered approach to participation, balancing areas of expertise, resource availability and potential for impact to be considered.
How Clinical Trials work
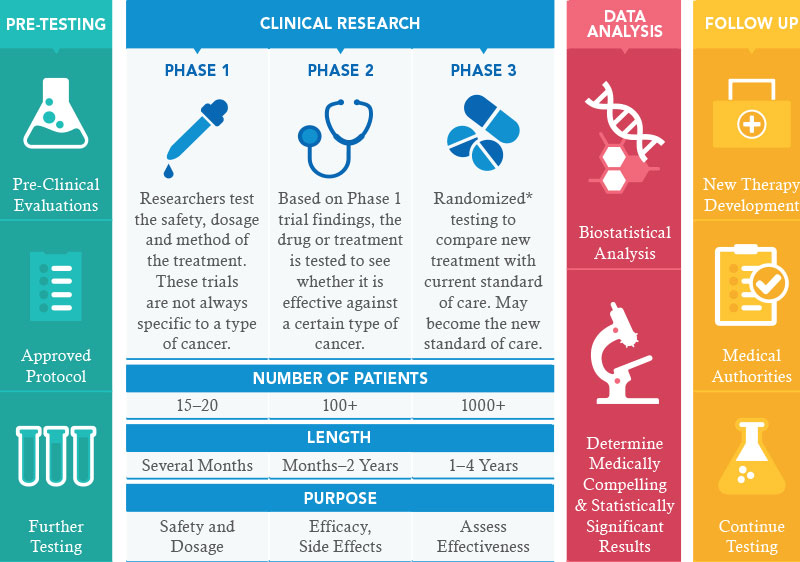
This infographic provides an overview of the three stages of clinical trials. Each trial begins with pre-testing, followed by research, data analysis and follow-up.
-

Phase l
Learn morePhase l clinical trials are the bridge between laboratory and clinic. They represent the first time a promising new agent, which has been tested extensively in the lab, is given to humans. The purpose is to understand what the drug does to the body and what the body does to the drug. The goal is to determine whether it’s safe and whether there is any hint of activity to warrant further development. -
Phase ll
Learn moreThe next phase of testing is Phase ll trial where the drug, having gone through Phase l, we have established the recommended dose. The main reason we do this trial is to see whether or not there is any clinical efficacy. A new treatment is tested in a larger group of people (up to 100), to determine if the new treatment has an effect on a certain cancer, and to learn how the new treatment affects the human body.

-

Phase lll
The next phase would be a Phase lll trial, where we compare it with what we have as standard treatment for that particular cancer, to see whether this new drug is any better than what we currently have. Typically, if the phase 3 trial shows an advantage of the new drug over the current treatment. The drug then obtains approval from various health authorities around the World and is entered into the market.
-

Phase l
Learn morePhase l clinical trials are the bridge between laboratory and clinic. They represent the first time a promising new agent, which has been tested extensively in the lab, is given to humans. The purpose is to understand what the drug does to the body and what the body does to the drug. The goal is to determine whether it’s safe and whether there is any hint of activity to warrant further development. -

Phase ll
Learn moreThe next phase of testing is Phase ll trial where the drug, having gone through Phase l, we have established the recommended dose. The main reason we do this trial is to see whether or not there is any clinical efficacy. A new treatment is tested in a larger group of people (up to 100), to determine if the new treatment has an effect on a certain cancer, and to learn how the new treatment affects the human body.
-

Phase lll
The next phase would be a Phase lll trial, where we compare it with what we have as standard treatment for that particular cancer, to see whether this new drug is any better than what we currently have. Typically, if the phase 3 trial shows an advantage of the new drug over the current treatment. The drug then obtains approval from various health authorities around the World and is entered into the market.
-
I could never have envisioned building a program quite like this,” Lillian admits. “Everybody feels such purpose. They know what they’re doing is important and they don’t want to fail anyone: patients, colleagues, or the program. They don’t want it to lose its cohesiveness, its quality. It’s definitely considered one of the top programs, not just in terms of science and research, but in terms of operational efficiency, quality and high standards. And yes, I’m saying this because it’s ours, but I think if you asked our peers, they would say the same.
 Dr. Lillian SiuDirector Phase l Program
Dr. Lillian SiuDirector Phase l Program -
The Bras family’s generosity and commitment to new drug development has helped pave the way to more personalized cancer treatment, which is the focus of the Princess Margaret’s Billion Dollar Challenge for Personalized Cancer Medicine.
 Greg LichtiVice President Development, The Princess Margaret Cancer Foundation
Greg LichtiVice President Development, The Princess Margaret Cancer Foundation -
We all know someone who has been affected by cancer, and the BRAS Drug Development Program, under the directorship of Drs. Malcolm Moore, Amit Oza, Lillian Siu, Philippe Bedard and Albiruni Razak, has achieved credibility on an international scale, and the advancements being made will bring us that much closer to finding a cure for this dreaded disease…
 Madam Hazel McCallionHonorary Chair of the BRAS DDP
Madam Hazel McCallionHonorary Chair of the BRAS DDP -
The BRAS Drug Development Program is one of the largest and most recognized drug development programs in Canada. Over the years, this remarkable program has witnessed substantial team growth and brought renewed hope to patients who have exhausted other treatment options. The lives of cancer patients have been improved, and even saved, thanks to you.
 Dr. Peter PistersPresident and CEO of University Health Network
Dr. Peter PistersPresident and CEO of University Health Network -
The Bras Family was remarkably visionary in establishing this center, and Canadian cancer patients are certainly indebted to the family for their generosity.
 Robert S. BellMDCM, MSc, FACS, FRCSC-President and Chief Executive Officer, University Health Network (Toronto)
Robert S. BellMDCM, MSc, FACS, FRCSC-President and Chief Executive Officer, University Health Network (Toronto)
